ClearLight Resources
Downloads, Links and More
A collection of resources to help the research community
Resources to Empower Discovery
This page is a collection of resources to help the research community better understand ClearLight Biotechnologies' history, technology, services, and path forward. Also, please visit our Frequently Asked Questions (FAQs). If you don’t see what you are looking for, please contact us.
Our mission is to revolutionize the diagnostic, prognostic, and predictive treatment of disease by enabling next generation technologies for non-destructive processing and digitally analyzing tissue in 3D. We are developing an automated instrumentation platform based on the CLARITY lipid-clearing technique.
Empowering Researchers
California Pharmaceutical and Biotechnology companies have more than 1,300 potential therapies in the pipeline that have the potential to help people facing cancer, infectious diseases, rare and genetic diseases and other disease states. Countless more potential drugs are in the early stages of discovery and research phase. ClearLight aims to empower researchers in the drug discovery process to see more biology so they can explore further.
Creating Cures Community
We applaud the research community and their involvement in the drug discovery and development process. These are the modern heroes who will create the cures of tomorrow for the disease(s) that impact human potential and decrease lifespan. Please join our Creating Cures community. You’ll receive our email newsletter periodically and have an opportunity to have your voice be heard as well.

ABOUT THE INNOVATORS
Meet the Inventor
Dr. Karl Deisseroth, professor of bioengineering and psychiatry at Stanford University, founded ClearLight based on the CLARITY lipid-clearing technique he and colleagues invented at Stanford University. Dr. Deisseroth has a long list of publications, honors and awards, including the 2015 Lurie Prize in Biomedical Sciences for the development of CLARITY and optogenetics. His scientific achievements have spawned additional research and inventions that have revolutionized the study of the brain and have led to major advances in neuroscience and biomedical engineering. He continues to serve ClearLight Biotechnologies as a Scientific Advisor. Follow Dr. Deisseroth on twitter at @KarlDeisseroth.

Who is ClearLight Diagnostics?
ClearLight Diagnostics was the former name for ClearLight Biotechnologies. The new name more accurately reflects the current short and longer-term business plan, as well as the future potential commercialization plan to introduce a Research Use Only (pharmaceutical and academics) platform into the market.
CLARITY Tissue Clearing Genesis
The CLARITY method was originally developed at the Karl Deisseroth Lab at Stanford University. It was first made public in a paper published in Nature in 2013. [ K Chung, J Wallace, S-Y Kim, S Kalyanasundaram, AS Andalman, TJ Davidson, JJ Mirzabekov, KA Zalocusky, J Mattis, AK Denisin, S Pak, H Bernstein, C Ramakrishnan, L Grosenick, V Gradinaru, and K Deisseroth. Structural and molecular interrogation of intact biological systems. Nature (2013) 497: 332-337.]
ClearLight Biotechnologies was founded in 2015 by Karl Deisseroth MD, PhD, the inventor of CLARITY, Optogenetics, and STARmap technologies. It is an early stage company focused on developing automated instrumentation and associated reagents to simplify and expedite non-destructive 3D tissue analysis.. The platform will enable an end-to-end solution for 3D analysis of preclinical and clinical models of disease.
A World of Opportunity
ClearLight Biotechnologies has an exclusive, worldwide license agreement with Stanford University for development and commercialization of CLARITY for diagnostic and clinical applications. We are committed to a future where researchers and clinicians worldwide have access to automated instruments bringing the power of CLARITY to clinical applications. Imagine a new 3D platform that empowers better clinical diagnosis, prognosis, and predictive applications, and is utilized in every research and pathology laboratory around the world. If you want to collaborate in bringing this opportunity to fruition, you can talk to our scientific staff.

Making Brains Transparent
Scientists can make brains transparent, so they can be labelled with molecular markers and imaged. The technique, CLARITY, invented by ClearLight Founder & Scientific Advisor, Karl Deisseroth, MD, PhD., enables the 3D visualizations seen in this Nature video.
The Future Has Arrived
Yesterday's science fiction is becoming science fact. It is now possible to trace the path of a single nerve projection through a forest of other cells. Researchers can illuminate the tissue microenvironment in a 3D volume. This see-through tissue clearing process can be integrated into a normal tissue processing workflow and be complementary with current 2D FFPE technologies to further empower researchers involved in pre-clinical and clinical research studies to see more biology.
We Value Our Relationships
We're looking for early adopters who value the potential of a 3D revolution in immunohistochemistry (IHC). ClearLight’s collaborative and pilot lab services will include tissue clearing using the CLARITY method, immunostaining, 3D imaging (multiplexed fluorescence), and we are developing the capability to perform true 3D tissue analysis in 3D. The team continues to develop our capabilities and would love to work with you to address your research needs. Contact us to discuss your project with our team of scientists.

LEARN MORE ABOUT CLARITY
CLARITY Tissue Clearing Process
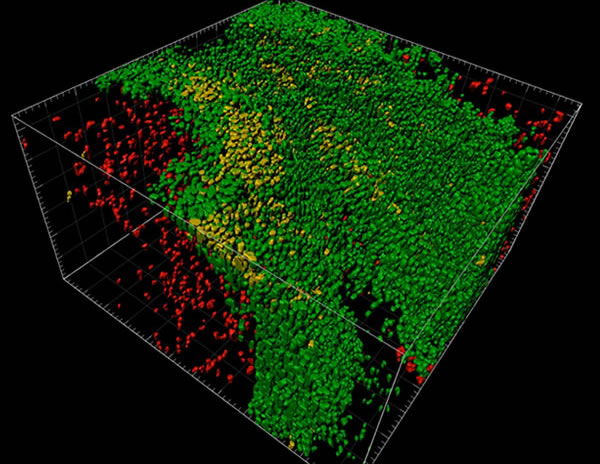
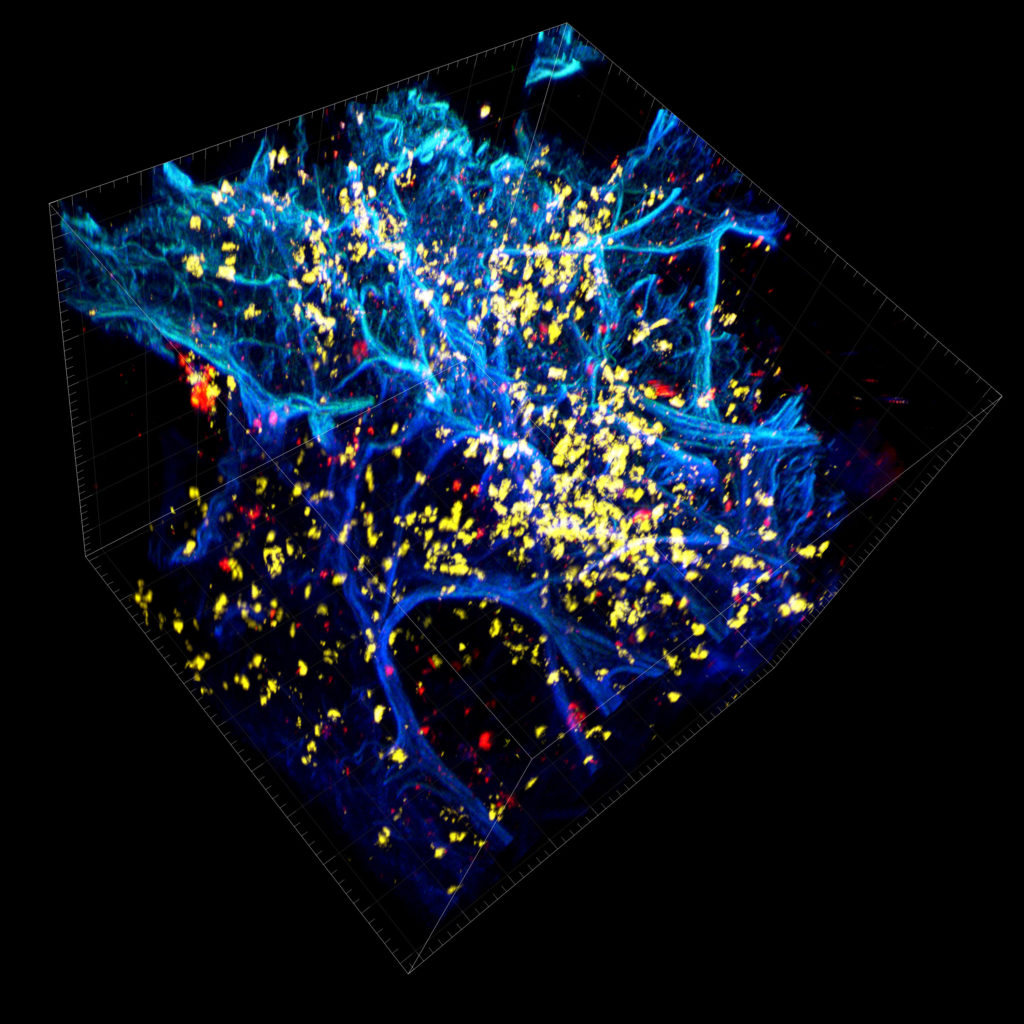
CLARITY was born from the need to overcome the opacity of lipids in brain tissue, which caused light to scatter during microscopic visualization of neurons, thereby obscuring image quality. CLARITY allows a tissue sample to be rendered optically clear, retain its three-dimensional integrity and allows for tissue staining and re-interrogation multiple times. It works for human and mouse brain tissue and most other intact human and rodent biological tissues.
Creating See-through Tissues
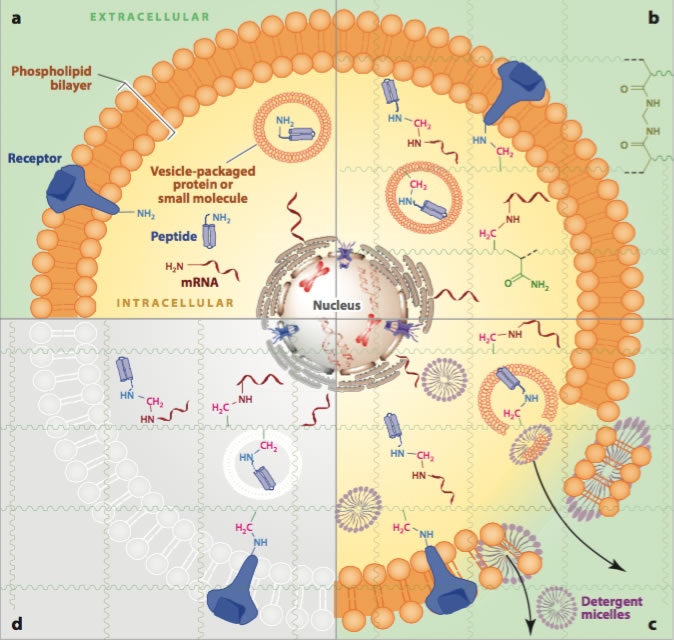
In the CLARITY method, a tissue sample is first embedded in a hydrogel. This hydrogel provides a three-dimensional matrix that supports the tissue sample and retains the structural integrity and spatial orientation of the critical tissue components. The non-critical elements such as lipids are removed using detergent solutions. Light can then penetrate the tissue sample rendering it “see-through” or transparent and thus permeable to molecules.
Tissue Staining
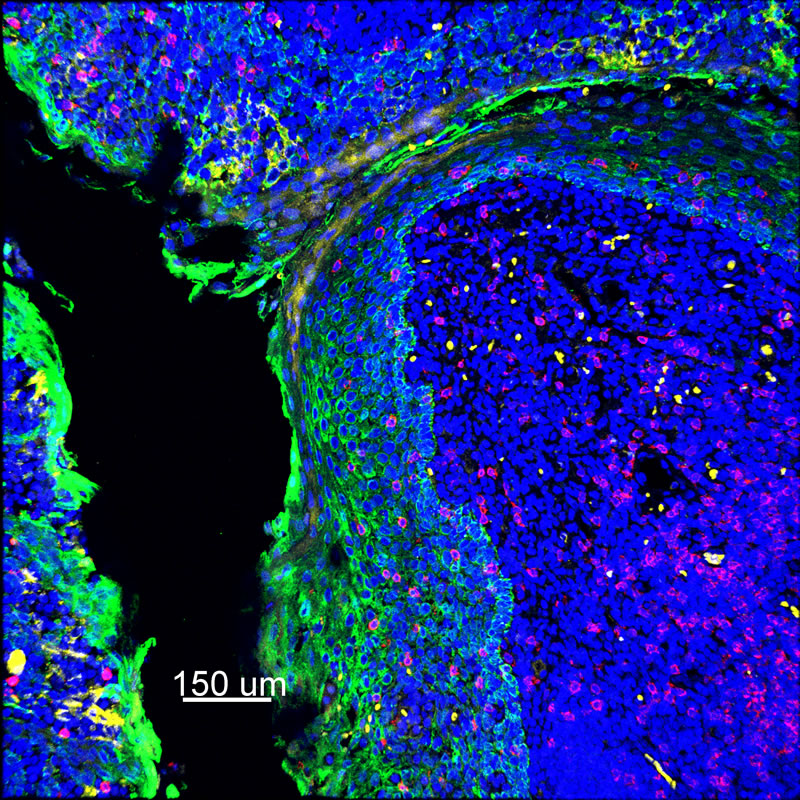
Researchers employ multiplexed IHC staining to identify cell phenotypes of interest within the tissue microenvironment. Since the sample is embedded and intact within the cleared hydrogel matrix, the stained tissue has the potential to be imaged entirely in three dimensions. The tissue’s transparency enables 3-D imaging of subcellular components such as DNA, RNA, and proteins for analysis of heterogeneous cellular interactions within the tumor microenvironment of a tissue.
CLARITY Advantage over Creating 3D Images from 2D Slices
2D thin section tissue imaging requires tissue destruction and digital reconstruction. Alternatively, the CLARITY methodology is non-destructive and allows the tissue to remain intact enabling a scientist to spatially identify the interaction of a variety of cellular phenotypes within the same tissue sample.
Passive Clearing versus Active Clearing
Passive tissue clearing with CLARITY is a straightforward and inexpensive, but a much slower process compared to active clearing. Active tissue clearing utilizes electrophoresis to apply electric fields to accelerate the removal of lipids from tissues and is more suitable technique for future clinical applications that require a shorter turnaround time.. The 3D tissue clearing technology, CRYSTAL, invented by Professor Adriano Aguzzi, M.D, Ph.D., and Daniel Kirschenbaum, M.D, Ph.D. at the University of Zurich and the University Hospital Zurich has the advantage of using a focused detergent-ion stream tailored to the sample to be optically cleared. Previous detergent electrophoretic methods for optical clearing, rely on an electric current irrespective of sample morphology.
Thus, in these approaches the ion stream traverses the sample container along the path of least electrical resistance, which is simply the surrounding buffer solution. Modifying this with the CRYSTAL technology, to a focused electrophoretic approach enhances the clearing process multi-fold and allows the clarification of a 1 mm thick tissue biopsy in 30 minutes. CRYSTAL is easy to miniaturize and parallelize, and it has been successfully utilized in the academic setting to test multiple drugs in animal models of disease.
Multiplex Immunohistochemistry (IHC) staining
IHC Staining is the general term used to describe the use of colored, often fluorescent, compounds that bind to very specific antigenic sites within a tissue sample. It is useful, because it can stain and provide visual information confirming the presence and precise location of a wide array of specific antibodies - that can only be present as a reaction to a specific antigen — thus the detection of the presence of specific antibodies through IHC staining is an important diagnostic tool.
IHC staining has been utilized as far back as the 1940s and is now routinely used in diagnostic medicine, bio-medical research, and histopathology.
In Multiplex IHC Staining, multiple sites within a single tissue sample are stained at once, with different colors representing different targets, or different cell types. This is especially helpful in the field of immuno-oncology as it allows for imaging the complex variety of cells within a tumor microenvironment.
ClearLight Biotechnologies in the News
What Others are Saying about CLARITY

“CLARITY is powerful. It will enable researchers to study neurological diseases and disorders, focusing on diseased or damaged structures without losing a global perspective. That’s something we’ve never before been able to do in three dimensions.” Francis Collins, Director of the National Institutes of Health (NIH)

“In surgical pathology, time is of the essence. By doing away with paraffin embedding and microtome cutting, the CRYSTAL technology allows for acquiring holographic ultra-high-resolution images of specimens more quickly than with conventional histology,” Professor Aguzzi, Director of the Institute of Neuropathology.

“The CLARITY technology holds promise to revolutionize spatial analysis by enabling 3D analysis of thick (up to 1000-micron) tumor to achieve more biologically relevant information regarding tumor heterogeneity and key spatial relationships in the tumor microenvironment.” Sunil Badve, MD - Joshua Edwards Professor of Pathology and Laboratory Medicine and Director of the Translational Genomics Core.